-Knowledge Base-
Methods for Cosmetics and Pharma
Selecting & Validating Analytical Methods for the Cosmetics and Pharmaceutical Industries
Neil Chapman, President
Deena Conrad-Vlasak M.Sc. Business Development
Preface
As a laboratory organization we are frequently asked should I test my new product as a drug or as a cosmetic; a relatively simple question but not a simple answer as is many cases the answer is both.
The FD&C Act (Federal Food, Drug & Cosmetics Act) defines cosmetics as: “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body…for cleansing, beautifying, promoting attractiveness, or altering the appearance” [FD&C Act, sec. 201(i)].” Typical examples would be lipsticks, perfumes, facial make up, etc. The law defines drugs however as: “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals” [FD&C Act, sec. 201(g)(1)].”

While simplistic in the descriptions a number of products, both Over the Counter (OTC) and Prescription based can fall into both categories; shampoo would be an example. While a shampoo claiming to make hair shiny or softer can be listed as a cosmetic requiring minimal oversight, a shampoo claiming to cleanse hair while treating dandruff is now considered both a cosmetic and a drug, requiring oversight from the FDA and falling under the guidelines of cGMP (current Good Manufacturing Practices) for both production and testing. (There are currently no cGMP requirements for cosmetics although this is being reviewed by the FDA, the European Union, Japan & Canada with the adoption of ISO Standard 22716 being considered).
The Requirements for Validated Methods for the Pharmaceuticals Industry
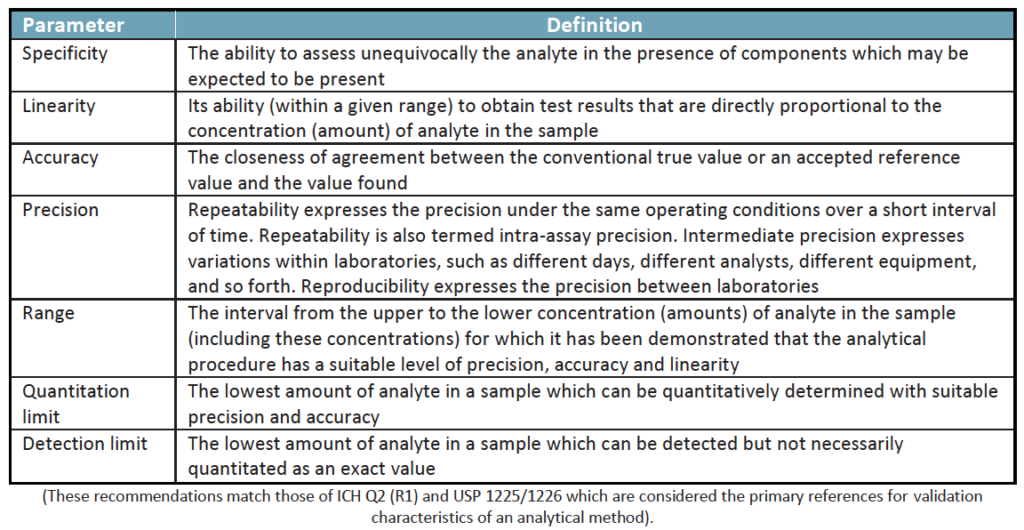
Validation of an analytical method shows the process that it establishes, but the collection of test data shows that the performance characteristics of the method meets the requirements for the intended application. Under the more stringent controls of cGMP, analytical methods need to be developed and proven for each of the active analytes of interest. Based on the nature of the test this might include such parameters as:
The JRC report commissioned by the European Union (2015) highlights the lack of standardized methods in the cosmetics industry due to the large variety of ingredients and formulations which creates issues due to matrix complexity and variability. ISO standard 22716:2007 (Cosmetics – GMP – Guidelines on Good Manufacturing Practices) makes manufacturers responsible for their products at all stages of production and EC regulation 1223/2009 establishes that: “The sampling and analysis of cosmetic products shall be performed in a reliable and reproducible manner”; it continues: “In order to ensure that testing results related to official controls are sufficiently robust and reliable, the analysis should be performed in accordance with the principles laid down in ISO 17025:2005 – General requirements for the competence of testing and calibration laboratories”.
Does the testing lab of choice need ISO 17025 accreditation?
Many labs working under the 17025 guidance have chosen to do so based on high volumes of similar samples with known parameters where method validation can be completed on an infrequent basis. Laboratories operating under cGMP for the Drug and Medical Device sector are more likely to operate following the guidelines listed in the JRC report due to the constantly changing matrices and active ingredients they experience.
Comparison between ISO, ICH and USP
With so many standards to consider a company needs to evaluate what level of validation is required, at what stage of production and under which regulatory authority. Methods need to be validated, verified, or revalidated in the following instances:
• Before initial use in routine testing
• When transferred to another laboratory
• Whenever the conditions or method parameters for which the method has been validated change (for example, an instrument with different characteristics or samples with a different matrix) and the change is outside the original scope of the method.
During method validation, parameters and acceptance criteria for system suitability checks and quality control checks should be defined. All analytical procedures or methods used for cGMP testing in a regulated laboratory must be validated. The table below considers the same parameters for cosmetics as we saw above for use in the pharmaceutical field; and highlights what is required under each international standard:
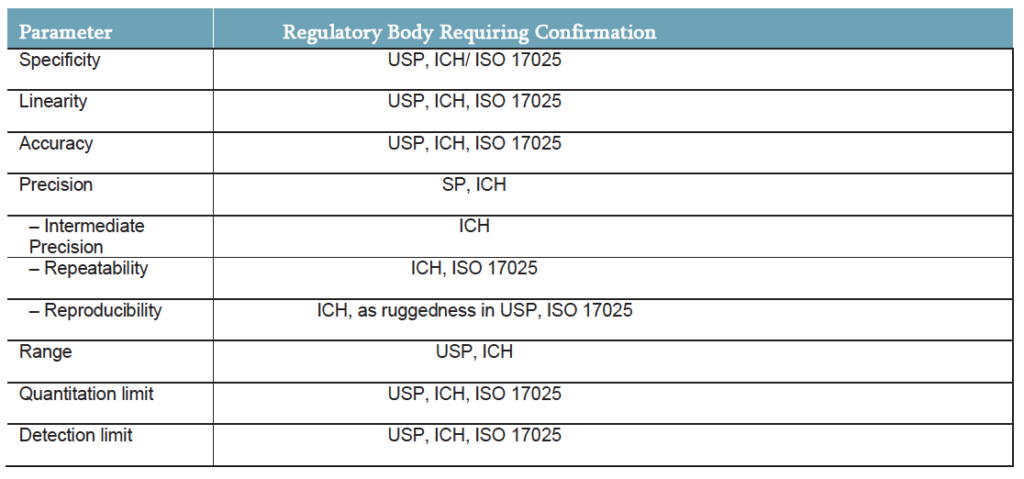
The requirement to comply with cGMP for the pharmaceutical industry has been long established, but with more and more products entering the gray space of cosmetic/pharmaceuticals the likelihood (as we can see from the European report) is that cGMP will ultimately become a requirement in the cosmetic sector as well. The adoption of either USP, ICH or ISO guidelines dictates the need to perform analysis in accordance with the table above in order to generate legally defensible data to protect consumers and client brands alike.
